What is direct immunofluorescence?
Direct immunofluorescence (DIF) is a technique used in the laboratory to diagnose diseases of the skin, kidneys, and other organ systems. It is also called a direct immune fluorescent test or primary immunofluorescence
DIF implies the application of antibody–fluorophore conjugate molecules to patient tissue samples obtained from biopsies. These antibodies-fluorophore conjugated abnormal target bowel movements protein in the patient's tissue. When exposed to light, the fluorophore emits its own frequency of light, seen under a microscope. The particular staining pattern and type of abnormal protein. statement seen in the tissue sample helps to diagnose the disease.
Direct immunofluorescence for the diagnosis of skin diseases.
DIF is useful in the diagnosis of suspected autoimmune disease, connective tissue diseases and vasculitis. The staining patterns observed in tissue samples may be specific to a disease entity or may need to be interpreted with the clinic and histological recommendations.
Autoimmune bullous diseases
DIF is useful in diagnosing the following autoimmune bullous diseases:
- Pemphigus vulgaris
- Leaf Pemphigus
- Paraneoplastic pemphigus
- Immunoglobulin (Ig) A pemphigus
- Bullous pemphigoid
- Gestational pemphigoid
- Mucous membrane pemphigoidscar pemphigoid)
- Acquired epidermolysis bullosa
-
Bullous systemic lupus erythematosus (SLE)
- Linear IgA bullous disease
- Dermatitis herpetiform
Connective tissue diseases
DIF is useful in diagnosing the following connective tissue diseases:
-
Systemic lupus erythematosus, discoidand subacute cutaneous shapes)
- Neonatal lupus erythematosus
- Systemic sclerosis
- Mixed connective tissue disease
-
Dermatomyositis
Vasculitis and other conditions.
DIF is useful in the diagnosis of cutaneous vasculitis and some other types of inflammatory Skin illness:
- Small pot (hypersensitivity) vasculitis
- Henoch - Schönlein purple
- Lichen planus
- Cutaneous porphyria delays
- Some adverse skin reactions to drugs.
- Photosensitivity rashes
Site of biopsy
The optimal site to take a skin biopsy depends on the suspected disease.
- Autoimmune bullous diseases: takes on a normal appearance perilesional skin within 1 cm of a bulla. Since false negative results can arise from samples from the lower extremities, avoid these sites if possible.
- Connective tissue diseases: the biopsy should be taken from a injury (often in areas exposed to the sun), ideally over 6 months old but still active. An additional sample is often taken in a place protected from the sun.
- Vasculitis: For best results, take a puncture biopsy or a deepshaving biopsy from an injury that is less than 24 hours old.
Another routine hematoxylin-eosin (H&E) biopsy is usually taken. histology. Note that the optimal biopsy site and required time differ for these examples.
- Autoimmune bullous diseases: remove an integer vesicle or biopsy of the edge of a blister.
- Vasculitis- Select a purple lesion less than 72 hours long.
Transport and storage of the biopsy sample.
Samples for DIF should not be placed in formalin, as this alters the proteins and significantly decreases the precision of the results. Transport the sample to the laboratory as soon as possible. Options for transporting the sample include:
- Placed in a salinesoaked gauze
- In saline solution solution
- In special media (eg, Michel or Zeus)
- Frozen in liquid nitrogen (sample should not be allowed to thaw).
Each laboratory has its own protocols. Samples in saline solution can produce higher sensitivity over other media if processed within 24-48 hours.
What happens to the sample in the laboratory?
DIF can be carried out on an automated machine or manually. The process involves first making frozen sections and then carrying out immunofluorescence.
Preparing frozen sections
- A needle biopsy is transported to the laboratory on gauze soaked in saline.
- The sample is placed in a gel.
- Liquid nitrogen is used to freeze the sample and the gel.
- The frozen sample is contained within the frozen gel.
- The sample can be stored in liquid nitrogen for about a week.
- Slices 4–6 microns thick are cut.
Preparing frozen sections
Put biopsy on gauze soaked in saline solution

Gel sample

Liquid nitrogen storage

Frozen specimen

Sample in liquid nitrogen

Cut thin slices of skin
Carrying out direct immunofluorescence.
- Five or six slides are made; each one for a different reagent. A slide will be used for normal H&E staining.
- A special pen is used to draw a perimeter to keep reagents inside the slides.
- The slides are washed.
- The reagents are composed.
- The reagents (antibody-fluorophore are conjugated with IgG, IgM, IgA, complement C3 protein, and when required fibrinogen) are dropped onto the slides and the slides are left for a time in the dark.
- The slides are washed again in solution.
- Glass covers are placed over the slides.
Carrying out direct immunofluorescence.

Five slides are made.

A special pen is used.

Slides washed in solution

Preparing reagents

Reagents

Reagent dropped on slides

Wash in solution

Prepare for the coverslip

Glass cover
Interpretation of direct immunofluorescence.
The prepared immunofluorescence slides are examined using a pathologist to determine primary immune deposition sites (if any), classes of immunoglobulin or other immune deposition, and deposition patterns. The staining patterns can be classified into five groups:
- Intercellular surface staining pattern (ICS)
- Linear basement membrane area (BMZ) pattern
- BMZ granular pattern
- Shaggy BMZ pattern
- Vascular and other patterns.
Immunofluorescence patterns in skin diseases.
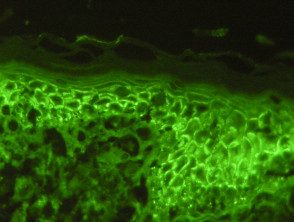
Pemphigus vulgaris
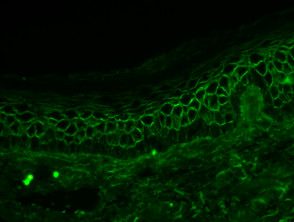
Leaf Pemphigus
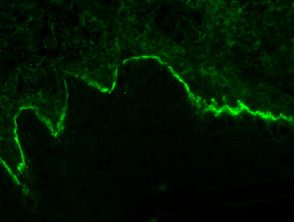
Bullous pemphigoid
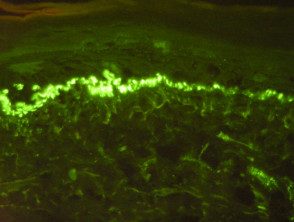
C3 in the area of the basement membrane
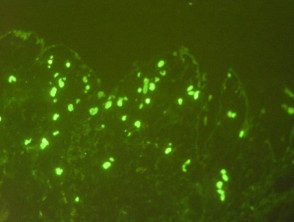
Vascular staining
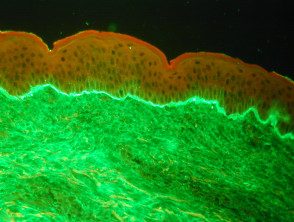
Linear IgA bullous dermatosis
Examples of staining patterns.
- Staining pattern of the intercellular space (chicken wire), pemphigus vulgaris.
- Staining pattern of the intercellular space (chicken wire), pemphigus foliaceus.
- Linear deposition of IgG in the area of the basement membrane, bullous pemphigoid.
- Linear deposition of IgA in the area of the basement membrane, linear bullous IgA skin disease.
- Vascular staining pattern in dermal vessels for IgM.
- Granular deposition of C3 in the area of the basement membrane
Staining patterns of specific skin diseases.
Pemphigus vulgaris
- ICS IgG standard (90–100%) or C3
Leaf Pemphigus
- ICS IgG or C3 standard
Paraneoplastic pemphigus
- Weak ICS pattern
- Linear or granular BMZ IgG or C3
- Sometimes, lichenoid change
Pemphigus IgA
- ICS with IgA
Bullous pemphigoid
- Linear BMZ with IgG and linear BMZ with C3
Mucous membrane pemphigoid (scar pemphigoid)
- Linear BMZ with IgG, C3 and IgA
Acquired epidermolysis bullosa
- Linear BMZ IgG and C3
- Sometimes linear BMZ IgA or IgM
Herpetiform dermatitis
- Granular BMZ with IgA
- Sometimes BMZ C3 granular
- Rarely, BMZ granular IgG and M3
Lupus erythematosus
- Systemic lupus erythematosus
- Granular BMZ IgG, IgM, IgA, C3
- Mottled epidermal nuclei IgG pattern (10-15%)
- Discoid lupus erythematosus
- Granular BMZ IgG, IgM
- IgM and IgA cytoid bodies
- Subacute cutaneous lupus erythematosus
- Granular BMZ IgG, IgM, C3
- Epidermal intracytoplasmic IgG particle deposition
- Cytoid bodies IgM, IgA
Systemic sclerosis
- Granular BMZ IgM
- Mottled deposition of epidermal nuclei (20%)
Mixed connective tissue disease
- Granular BMZ (15%)
- IgG speckled epidermal nuclei pattern (46–100%)
Dermatomyositis
- Granular IgM, IgG, C3
- Cytoid bodies IgM, IgA
Lichenoid reactions (lichen planus, drug reactions, and photodermatosis)
- Shaggy BMZ Standard for Fibrinogen
- Cytoid bodies IgM, IgA
Cutaneous porphyria delays
- Dermal vessels: continuous thick staining IgG, IgA, C3
- Granular BMZ C3, IgM
- Linear BMZ IgG, IgA
Vasculitis
- Strong dermal vessels IgM, IgG, C3, fibrinogen
Henoch-Schönlein purpura
- Strong dermal IgA vessels
How reliable are direct immunofluorescence results?
The DIF test is moderately sensitive in detecting a variety of diseases. The sensitivity for DIF approaches 100% for the group of diseases of the pemphigus, and the sensitivity has been reported to be 55-96% for bullous pemphigoid. A study of ten patients with Henoch-Schönlein purpura and nine with lupus erythematosus demonstrated positive DIF tests in all patients.
The number of false negative results with DIF depends on the quality of the sample, the laboratory processing, and whether the patient has been in treatment or not. Reasons for false negative results include:
- An incorrect biopsy site
- Lack of epidermis in biopsy and other technical errors
- Prolonged storage of the sample before transport to the laboratory.
- Photobleaching and other lab errors.
- The patient is on active immunomodulatory treatment.
