What are cryopyrin-associated periodic syndromes?
Periodic associated with cryopyrin syndrome or syndromes (CAPS), also known as cryopyrinopathies, are genetic autoinflammatory syndromes defined by “gain of function” mutations affecting the protein cryopyrin.
Three distinct clinical syndromes are recognized within CAPS.
- Family Cold autoinflammatory syndrome (FCAS)
-
Muckle-Wells syndrome (MWS)
- Neonatalmulti-system boot inflammatory disease/chronic child neurological cutaneous and joint syndrome (NOMID / CINCA)
The mildest form is FCAS, and the most severe form, NOMID/CINCA, is often fatal. Clinical overlap is now recognized, suggesting that these are a disease continuum.
Some cases of Schnitzler syndrome may also fall into this category.
Who gets cryopyrin-associated periodic syndromes and why?
The periodic syndromes associated with cryopyrin are all autosomal dominant genetic conditions, meaning that only one copy of the abnormal gene It is required to develop the clinical syndrome. FCAS and Muckle-Wells syndrome are usually familial, inherited from an affected parent. NOMID/CINCA appears as spontaneous mutation, as it causes severe disabilities and is often fatal before adulthood.
Patients with overlapping features are increasingly reported to:
- between FCAS and MWD
- between MWD and mild NOMID/CINCA
- but not between FCAS or MWS and NOMID/CINCA serious.
Examples include sensitivity to cold, frequent headaches, or asymptomatic papilledema (swelling at the back of the eye) in MWD or deafness in FCAS. Even within a family, members can be classified as having different syndromes based on clinical characteristics.
Molecular biology and genetics
All three conditions are caused by mutations in the same gene, NLRP3, Located in chromosome 1 (1q44). Approximately 100 different mutations, mostly missense mutations in exon 3 of this gene, have been identified in patients with cryopyrin-associated periodic syndromes. Missense mutations in exons 4 and 6 have rarely been identified. Certain mutations result in a specific clinical syndrome, for example, Y570C, Y570F, F309S, F523L are only reported in severe NOMID/CINCA. Other mutations (e.g., R260W, V198M, V262G, D303N) have been identified in more than one clinical picture, even within a family. This would suggest that there may be additional influences, genetic or environmental, that affect clinical presentation. Asymptomatic carriers, mainly in affected families, have also been detected.
the NLRP3 The gene is particularly expressed in white blood cells (especially neutrophils) and chondrocytes (cartilage cells). It encodes cryopyrin, a protein involved in the formation of inflammasomes. Inflammalamomas are protein complexes found inside cells. They are important in the innate immune system. Disease-related changes in cryopyrin result in the loss of a regulatory step that allows for greater activation of caspase-1 and therefore greater activation of interleukin (IL) -1. Activated IL-1β is a powerful stimulator of the inflammatory cascade. Expression of the NLRP3 gene in chondrocytes may be relevant to joint pain/inflammation in FCAS and MWS and bone overgrowth in NOMID/CINCA. The latter does not seem to respond to IL-1 receiver blocking. Expression in chondrocytes may also be involved in the development of sensorineural deafness in MWS and NOMID/CINCA.
The mechanism by which cold triggers attacks in FCAS remains unknown.
Not all clinically typical CAPS cases have had a NLRP3 identified genetic mutation, particularly the NOMID/CINCA form. Somatic Mutations, genetic mutations that occur during fetal development, have been found to explain a small number of apparently mutationally negative cases. the NLRP3 the mutation is detected in only a percentage of cells in the peripheral blood. Such tests are difficult and are currently only performed in a research setting.
What are the clinical characteristics of cryopyrin-associated periodic syndromes?
The clinical features common to these three conditions are intermittent or recurrent attacks that include:
- Fever
- Urticaria-I like it eruption (see below)
- Joint pain (arthralgia)
- Conjunctivitis.
In addition, each syndrome has its own distinctive clinical features (see the description of a specific syndrome).
What are the characteristics of the skin rash?
The urticaria-like rash is the same clinically and histologically in all forms of cryopyrin-associated periodic syndromes. Of trait:
- It's usually the first sign of the syndrome
- It develops at or shortly after birth, or in early childhood.
- It is migratory, meaning that the skin lesions move.
- Maculopapular or hives may appear.
- The symptoms tend to be atypical for hives, with itching, burning, warmth and/or tightness, rather than itching.
- Lesions last less than 24 hours.
- They often show a diurnal pattern, worsening at night.
- The intensity varies between patients and between episodes.
Periodic rash associated with cryopyrin syndrome
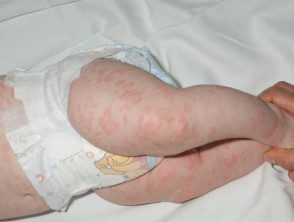
Periodic rash associated with cryopyrin syndrome
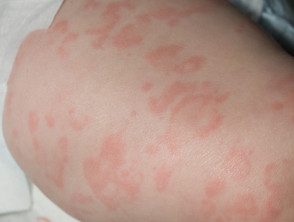
Periodic rash associated with cryopyrin syndrome
How are cryopyrin-associated periodic syndromes diagnosed?
The diagnosis of a cryopyrin-associated periodic syndrome should be considered in patients who present with recurrent episodes of fever, rash, joint pain, and inflammation of the eyes, without evidence of infection or autoimmune disease. Although these are all autosomal dominant conditions, a positive family history cannot always be obtained.
A delay in the diagnosis of a cryopyrin-associated periodic syndrome is common since all forms are rare. Initial diagnoses of viral infections and allergies are generally considered This delay in diagnosis, and therefore treatment, can lead to interleukin-1-induced organ damage, such as deafness and amyloidosis.
Acute phase reactants in blood, such as erythrocytes sedimentation rate (ESR), C-reactive protein (CRP) and serum amyloid A (SAA), increase markedly, even when the patient is well between episodes. Anemia of chronic diseases and a greater number of white blood cells, especially neutrophils, can be detected.
Skin biopsy of the urticaria-like rash may suggest the diagnosis of a cryopyrin-associated periodic syndrome, as it shows a perivascular and sometimes peri-eccrine (around sweat glands) neutrophilic infiltrate of the lattice dermis, without mast cells or vasculitis. This is quite different from normal hives (hives).
A commercial test is available to sequence exon 3 of NLRP3 gene. This will detect most, but not all, known mutations associated with cryopyrin-associated periodic syndromes.
What is the treatment of periodic syndromes associated with cryopyrin?
All three clinical syndromes respond very well to therapy with interleukin-1 antagonists, such as anakinra, rilonacept, and canakinumab. All patients with CAPS respond, provided a sufficient dose is administered.
Anakinra
The dramatic effectiveness of the biologic agent anakinra, an IL-1 receptor antagonist, has been demonstrated in a series of large clinical trials involving many patients with cryopyrin-associated periodic syndromes. It is administered daily. subcutaneous injection, and local injection reactions (itching, swelling, redness) are the most common adverse effects. Anakinra is the standard of care for NOMID/CINCA and was approved by the FDA for this indication in 2013. The dose range varies: 0.5–1.5mg/kg/d for FCAS, up to 3.5 mg/kg/d for MWS and up to at 10 mg/kg/day for severe NOMID/CINCA in infants.
Note: Anakinra is not registered or subsidized in New Zealand (March 2011). In other countries, such as the US and Europe, its registered indication is rheumatoid arthritis.
Rilonacept
Rilonacept was the first FDA-approved therapy for CAPS. It was approved in 2008 for FCAS and MWS in adults and children aged 12 years and older. Rilonacept is a dimeric fusion protein that binds to the interleukin-1 receptor accessory protein and the IL-1 type 1 receptor. It is administered weekly as a subcutaneous injection. Adverse events Mild to moderate severity, including local injection site reactions, upper respiratory tract infections, headache, joint pain, and diarrhea, has been reported in clinical trials. Two deaths have been reported during treatment with rilonacept for CAPS: one from pneumococcus meningitis and the other from coronary heart disease.
Canakinumab
Canakinumab has also been approved by the FDA (2009) for FCAS and MWS and has been approved for ages 4 years and older. In Europe, it has been approved for all forms of CAPS. Canakinumab is a completely humanized product. monoclonal antibody specifically directed against IL-1β. It is administered as a subcutaneous injection every 8 weeks. In a clinical trial with 35 patients with CAPS, the commonly reported side effects were nasopharyngitis, rhinitis, nausea, diarrhea and Vertigo. No local injection site reactions were reported. Two serious adverse events reported during the trials were acute angle vertigo glaucoma and recurrent lower urinary tract infection with septicemia. Vertigo may develop more frequently in patients with sensorineural deafness associated with some forms of CAPS.
A dramatic response is seen within hours or days after injection for daily symptoms (fever, rash, headaches, joint pain, conjunctivitis) and acute phase reactants in the blood. Improved long-term complications have also been reported in some patients with hearing loss, vision loss, amyloidosis (kidney disease), growth retardation, but not the bone abnormalities or existing mental retardation seen in severe NOMID/CINCA. There is evidence to suggest that starting treatment in very young children can prevent or minimize long-term neurological disability.
It has been recommended that long-acting anti-IL-1 agents in very young children be used in conjunction with antibiotics. prophylaxis and immunization against steotococci pneumonia and Haemophilus influenzae regarding posterior splenectomy.
Treatments that block the effect of IL-1beta have had a dramatic effect on the quality of life of those suffering from these rare cryopyrin-associated periodic syndromes.
