What is cobimetinib?
Cobimetinib (Cotellic™, Genentech Inc. California, USA) is a prescription drug used, in combination with vemurafenib, for the treatment of patients with melanoma.
Cobimetinib received US Food and Drug Administration (FDA) approval in the US in 2015. In April 2017, Medsafe approved cobimetinib for the treatment of melanoma patients in New Zealand.
Metastatic melanoma
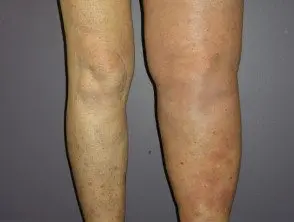
Metastatic melanoma
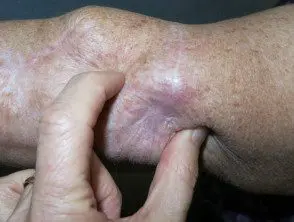
Metastatic melanoma
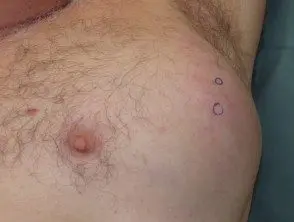
Metastatic melanoma
Who should take cobimetinib?
Cobimetinib, in combination with vemurafenib, is indicated for the treatment of patients with unresectable disease or metastatic melanoma, in which the Cancer that has spread to other parts of the body or cannot be removed by surgery. An indicator of this melanoma is an abnormal disorder. gene He called the BRAF V600E or V600K mutation, which must be confirmed in tumor samples before the start of treatment.
Cobimetinib is not indicated for the treatment of patients with a normal or wild type BRAF gene.
How does cobimetinib work?
Cobimetinib is a small molecule inhibitor that blocks MEK enzyme, a component of the kinase cascade in the mitogen-activated protein kinase (MAPK) pathway. Components of the MAPK pathways are frequently mutated in patients with evil one melanoma, particularly RAF isoform BRAF. Mutations of BRAF cause constitutive activation of these signaling pathways, which can lead to cancer.
Cobimetinib and vemurafenib target two different kinases in the MAPK pathway. Clinical trials have shown that the combination of cobimetinib with vemurafenib results in improved survival in melanoma patients harboring BRAF V600 mutation.
How is cobimetinib given?
Recommended dose
Cobimetinib is available in 20 mg tablets under the brand name Cotellic.
- The recommended dose is 60 mg once daily for the first 21 days of each 28-day cycle, continued until disease progression or intolerable. toxicity occurs.
- Cobimetinib can be taken with or without food.
- If a dose is missed or vomiting occurs when the dose is taken, resume dosing with the next scheduled dose.
Vemurafenib (ZELBORAF™) must be taken every 12 hours for each day in the 28-day cycle (no rest period). If a dose of vemurafenib is missed, it should be taken as soon as remembered. Do not make up for the missed dose if the missed dose is within 4 hours of the next scheduled dose.
Use of cobimetinib in specific populations.
Pregnant woman
There are no data available on the use of cobimetinib in pregnant women to inform of a drug-associated risk for major birth defects and miscarriage. Based on the findings of animal reproduction studies and its mechanism of action, cobimetinib may cause fetal harm. Pregnant women should be informed of the potential risk to the fetus.
Breastfeeding women
There is no information on the presence of cobimetinib in human milk or its effects on a breastfed infant. The potential benefit-risk should be considered when prescribing cobimetinib to the mother. Nursing women should be advised not to breast-feed during cobimetinib treatment and for 2 weeks after the final dose.
Children
The safety and efficacy of cobimetinib have not been evaluated in children.
Females and males with reproductive potential
Female patients of reproductive potential should be advised to use effective contraception during treatment with cobimetinib and for 2 weeks after the final dose. Based on findings in animals, cobimetinib may reduce fertility in females and males of reproductive potential.
Old people
Clinical studies with cobimetinib did not include sufficient numbers of individuals 65 years of age and older to determine whether they respond differently from younger subjects.
Individuals with hepatic or renal disability
No dose adjustment of cobimetinib is required in patients with mild (Child-Pugh A), moderate (Child-Pugh B), or severe (Child-Pugh C) hepatic impairment. not dedicated pharmacokinetics A trial has been conducted in patients with renal insufficiency. Dose adjustment is not recommended for mild to moderate renal impairment (creatinine clearance 30-89 ml / minute) based on the results of the population pharmacokinetic analysis. A recommended dose for patients with severe renal insufficiency has not been established.
What are the possible side effects of cobimetinib?
Common side effects include:
- Diarrhea
- Eruption
- Photosensitivity
- Nausea
- Stomatitis
- Fever
- Alopecia
- Thrombocytopenia.
Rare but potentially serious side effects include:
- Severe diarrhea leading to dehydration and kidney failure.
- Rhabdomyolysis
- cardiac toxicity
- Retinal detachment
- Embryo-fetal toxicity
- Joint pain (arthralgia)
hepatotoxicity
elevations in serum Aminotransferase and alkaline phosphatase levels are common during vemurafenib therapy, and are even more common when combined with cobimetinib.
Instances of clinically apparent liver damage with jaundice have been reported during clinical trials of cobimetinib and vemurafenib therapy, but the clinical features, course, and outcomes of these episodes have not been described in detail.
MAPK pathway inhibitors as a class are often associated with transient serum enzyme elevations and, more rarely, with cases of clinically apparent liver injury, but the clinical features have not been described and the association with cobimetinib has not been clearly defined. .
The rate of clinically significant liver damage and liver failure associated with protein kinase inhibitors is increased in patients with preexisting cirrhosis or liver failure due to liver tumor burden. The product label for cobimetinib recommends routine liver function test monitoring during treatment.
Serum aminotransferase elevations above five times the upper limit of normal (if confirmed) should lead to temporary discontinuation, which should become permanent if these laboratory values do not improve significantly or resolve within a few weeks.
There does not appear to be any cross-reactivity with other tyrosine kinase receiver inhibitors In some situations, it may be appropriate to switch to another protein kinase inhibitor.
Risk of new cancers.
Cobimetinib in combination with vemurafenib may cause new skin cancers. These may include:
- Cutaneous scaly cell carcinoma
-
Keratoacanthoma
- Basal cell carcinoma
Heart problems
Cardiomyopathy, defined as symptomatic and asymptomatic Decreased left ventricular ejection fraction (LVEF) may occur with cobimetinib.
The safety of cobimetinib has not been established in patients with base LVEF that is below the institutional lower limit of normal or below the 50%.
LVEF should be assessed before initiation of cobimetinib, 1 month after initiation, and every 3 months thereafter until drug discontinuation. Left ventricular events dysfunction must be managed through interruption, reduction or discontinuation of treatment.
Drug interactions
Cobimetinib is metabolized in the liver via the cytochrome P450 system, predominantly CYP3A, and is susceptible to drug interactions with potent inhibitors or inducers of this microsomal enzyme.
Co-administration of cobimetinib with itraconazole (a strong CYP3A4 inhibitor) increased cobimetinib systemic exposure by 6.7 times.
Concurrent The use of cobimetinib and strong or moderate CYP3A inhibitors should be avoided. If short-term (14 days or less) concurrent use of moderate CYP3A inhibitors, including certain antibiotics (eg, erythromycin, ciprofloxacin) is unavoidable (14 days or less), the cobimetinib dose should be reduced from 60 to 20mg
An alternative to a strong or moderate CYP3A inhibitor should be used in patients taking a reduced dose of cobimetinib (40 or 20 mg daily). After discontinuation of a moderate CYP3A inhibitor, cobimetinib should be resumed at the previous dose.
Co-administration of cobimetinib with a strong CYP3A inducer may decrease the systemic exposure of cobimetinib by more than 80% and reduce its effectiveness. Concomitant use of cobimetinib and strong or moderate CYP3A inducers, including but not limited to carbamazepine, efavirenz, phenytoin, rifampin, and St. John's wort, should be avoided.
Contraindications
Cobimetinib should be withheld in the following circumstances:
- A history of mental health problems, including suicidal thoughts, depression, anxiety, or mood problems.
- Concurrent infection that doesn't go away or keeps coming back
-
Tuberculosis (TB) or close contact with someone with TB
- Recently received or scheduled to receive a vaccine
- plan to get pregnant
-
Breastfeeding or plan to breastfeed.
