What are they chromosomes?
The chromosomes carry genetic material of a organism (reeoxyribonucleic Acid, DNA) There are 23 pairs of chromosomes in human cells. These chromosomes carry all the genetic information for each cell to function properly.
Chromosome abnormalities can cause a variety of genetic disorders that can lead to developmental delay, congenital defects and abnormal bodily function [2].
These genetic diseases may be due to the loss or gain of chromosomes or there are deletions or duplications of a chromosome. In these cases, the specific cell is missing genes so it cannot produce certain proteins and carry out its function correctly. Examples relevant to dermatology include:
-
Down syndrome (trisomy 21)
-
Turner syndrome (single functional X chromosome)
-
DiGeorge syndrome.
What is it cytogenetic tests?
Cytogenetics is the study of chromosomes and their structure. [1].
Cytogenetic testing involves the analysis of cells in a sample of blood, tissue, amniotic fluid, bone marrow or cerebrovascular fluid to identify any changes in an individual's chromosomes.
There are 3 main methods of cytogenetic testing.
- routine karyotype
- Fluorescent in the place hybridization (FISH)
- Comparative Genomic Hybridization (CGH) and microarray comparative genomic hybridization (aCGH)
Karyotype
The karyotype was one of the first methods of chromosome analysis. This method uses light microscopy and standardized staining procedures on cells in the metaphase portion of the cell cycle when chromosomes are most condensed [3].
to do the chromosomal análisis más efectivo y eficiente, se han desarrollado manchas para unirse al ADN y producir patrones de bandas característicos para identificar diferentes cromosomas [3]. La mancha más utilizada es el tinte Giemsa. [3]. A través de este proceso, los cromosomas se pueden organizar en un cariograma de 23 pares y cualquier anomalía que implique aneuploidía y grandes translocations can be identified.
Karyotyping can only identify changes in about 3 megabases [3]. Any abnormality involving less than this will not be detected by routine karyotyping. It can be used to identify Down syndrome and Turner syndrome.
Karyotype for Down syndrome
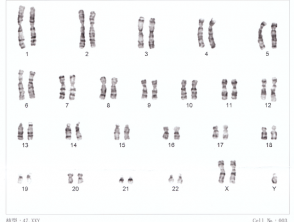
Human XXY Chromosomes
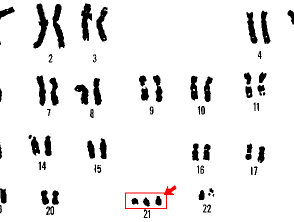
21 trisomy Down syndrome
Fluorescent in situ hybridization
Fluorescence in situ hybridization (FISH) was first introduced in the late 1980s and has rapidly become a well-known diagnostic cytogenetic test in both congenital and acquired diseases. [5]. FISH has much higher resolution than routine karyotyping [3], especially when used on interphase cells [6].
FISH uses fluorescent probes with complementary base sequences to locate the presence or absence of specific portions of DNA on chromosomes. [1.4]. The probe and target DNA must be denatured with heat or chemicals to break the hydrogen bonds in the DNA and allow hybridization to occur once the 2 samples are mixed. [6]. Fluorescent probes form new hydrogen bonds with their complementary base pairs in DNA, and this can be detected by microscopy. [6].
FISH is commonly used to detect specific chromosomal deletions or translocations associated with pediatric conditions or cancers [1]. Examples include the deletion of chromosome 22 in DiGeorge syndrome and the translocation from a gene on chromosome 22 and 9 on chronic myeloid leukemia [1].
FISH is also used to melanocytic lesions to distinguish atypical melanocytic naevi (eg Spitz nevus) since evil one melanoma. Add a brief description of this use and others relevant to dermatology.
Fluorescent in situ hybridization in childhood melanoma
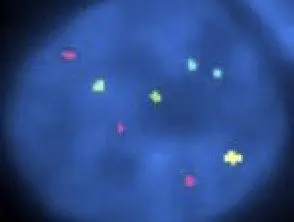
Childhood melanoma FISH pathology
Comparative Genomic Hybridization
Comparative genomic hybridization (CGH) is a method of molecular cytogenetic test that detects chromosomal copy number variants without the need for cell culture [7]. It was first developed to identify such changes in tumors [7,8].
CGH uses 2 genomes; the test sample and control, which are fluorescently labeled to differentiate between the two [8]. The two samples are denatured and mixed together, allowing hybridization of the metaphase chromosomes. The fluorescent signal intensity of labeled test DNA relative to control DNA can be plotted along each chromosome, showing the loss or gain of genetic material and allowing identification of any copy number. [8].
CGH differs from other cytogenetic testing methods in that it is not based on a specific target, nor does it require prior knowledge of the region under examination [8]. Instead, CGH can quickly scan a whole genome for these chromosomal imbalances [8] and is useful in cases where the diagnosis is unknown. A limitation of CGH is the size of the genetic alteration it can identify, the resolution of CGH is poor at approximately 5–10 megabases [8].
Comparative Genomic Hybridization
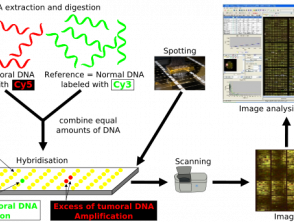
Array Comparative Genomic Hybridization Protocol
When is this method used in dermatology?
Microarray CGH uses a similar technique to CGH but provides much higher resolution by using microarray [8]. Small sections of DNA are used as targets for analysis; these sections are immobilized in solid support [8]. As in CGH, the DNA sample and control are fluorescently labeled to differentiate between them. The samples are mixed and added to the microarray where they compete to bind to the probes on the microarray [8]. The intensity of the different fluorescent signals can be assessed and small gains or losses within the DNA identified. A disadvantage of the CHG microarray is that it cannot detect balanced chromosomal structural changes, such as balanced translocations or inversions. [1].
When is cytogenetic testing indicated?
Cytogenetic tests are used when a genetic abnormality is suspected.
Prenatal tests in a high-risk pregnancy
Cytogenetic tests are performed on samples obtained in the womb via amniocentesis or by chorionic villus sampling to identify a fetus with chromosomal abnormalities, such as trisomy 21 in Down syndrome.
diagnostic tests
Cytogenetic testing is often used in pediatrics in an attempt to identify the underlying cause of developmental disorders or birth defects. A diagnosis can be a great relief to families of affected children and will allow advice on appropriate management and forecast.
Hematological Cancer
Cytogenetic testing is used in hematologic cancers such as chronic myeloid leukemia (CML) where a specific reciprocal translocation between chromosomes 22 and 9 results in the Philadelphia chromosome, which is present in 95% cases.
Which are the contraindications with cytogenetic tests?
There are no medical contraindications for cytogenetic testing.
Informed consent must be obtained before testing is arranged.
What are the benefits of cytogenetic testing?
Cytogenetic tests can offer diagnosis and help with long-term management of relevant diseases. It also allows genetic counseling for parents about risk in future pregnancies and, in some cases, guides the geneticist on whether to test other family members.
What are the disadvantages of cytogenetic tests?
Unfortunately, cytogenetic tests are limited by their resolution. Different methods can identify small gains and losses of genetic material, as well as larger translocations, but do not allow testing of a single translocation. nucleotide variations that could contribute to the patient's condition. There is also the possibility that cytogenetic testing will identify other chromosomal changes that are not necessarily related to the patient's condition.

