January 30, 2020: UK and Europe suspend the marketing of Picato® Gel.
The Regulatory Agency for Medicines and Health Products, United Kingdom [1] and the European Medicines Agency (EMA) [2] have announced that the marketing of Picato® Gel containing ingenol mebutate has been discontinued. They have recommended that patients stop using the product pending an investigation into its safety with regard to the skin. Cancer.
See more:
European marketing of Picato is suspended while the risk of skin cancer is reviewed. January 17, 2020. Medscape Medical News.
Class 2 Drug Recall: LEO Laboratories Ltd, Picato 150 mcg / g gel. January 27, 2020. Gov.UK
What is ingenol mebutate gel?
Ingenol mebutate (also called ingenol-3-angelate) is an extract from a common plant, small flowers, or milkweed (Euphorbia peplus) Ingenol mebutate is derived from a cultivar of Euphorbia peplus which is specifically grown in Queensland for this purpose.
It is useful in the treatment of actinic keratosis (actinic keratoses), which are rough spots caused by long-term sun exposure.
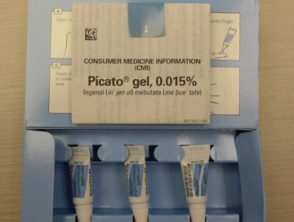
In January 2012, the United States Food and Drug Administration (FDA) approved ingenol mebutate gel for the treatment of actinic keratoses on the face, scalp, trunk, and extremities. Ingenol mebutate gel is available in concentrations of 0.015% and 0.05% and is manufactured by LEO Pharma under the brand name Picato®. Ingenol mebutate gel was registered as a prescription medication by MedSafe for use in New Zealand in October 2013.
The two or three day course of ingenol mebutate gel compares favorably with several weeks or months required for another current therapies used for actinic keratoses, such as 5-fluorouracil cream and imiquimod cream. The treatment can be repeated at a later date if necessary.
Ingenol mebutate gel
Effects of ingenol mebutate gel on facial actinic keratoses
Effects of ingenol mebutate gel on facial actinic keratoses
How is ingenol mebutate gel given?
The administration of ingenol mebutate gel is not recommended until the skin has healed with any previous medication or surgical treatment. It can be applied at any time of the year.
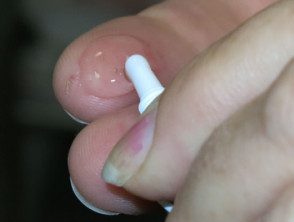
Ingenol mebutate gel is applied to a sun damaged area. The contents of a single dose tube will cover approximately 5 cm x 5 cm of skin. Treatment varies depending on the site of the keratosis.
- The gel must be refrigerated.
- Read the instructions on the box carefully.
- Ingenol mebutate gel 0.015% (150 mcg / g) is applied once a day for three days to the face and scalp.
- Ingenol mebutate gel 0.05% (500 mcg / g) is applied once a day for two days to the trunk and extremities.
- Hands should be washed thoroughly after applying the gel, so that it does not accidentally spread to other sites.
The treated area is allowed to dry for 15 minutes after application and should not be washed or touched for 6 hours after treatment. The treated area can be gently washed after that. Activities that cause excessive sweating should be avoided.
The treated areas become inflamed, often scab over, and then heal for a few days. If blisters form or ulceration occurs, the gel should not be reapplied at this site until the skin has completely healed. The moisturizer can be applied as needed when the skin peels off.
| Day 0 pretreatment | 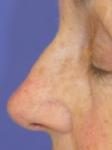 | 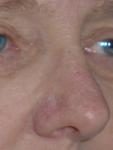 | 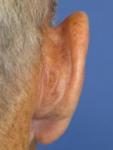 |  |
| The worst day |
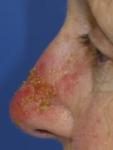 Day 2 |
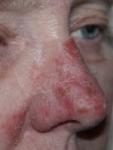 Day 5 |
 Day 4 |
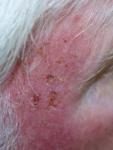 Day 3 |
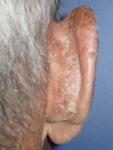
| Tracing |
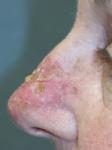 Day 6 |
 Day 13 |
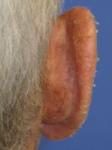 Day 7 |
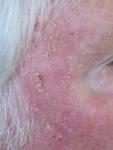 Day 7 |
How does ingenol mebutate gel work?
How ingenol mebutate works in actinic keratoses is incompletely understood. It seems to have a double mechanism of action:
- Quick injury necrosis
- Specific neutrophils-mediate, antibody–dependent cellular cytotoxicity
Link to key evidence from clinical trials
Possible pharmacological interactions with ingenol mebutate
Studies have shown that drug interactions are unlikely to be of clinical importance.
- Ingenol mebutate is metabolized by human liver cells.
- The ingenol mebutate does not inhibit or induce CYP450 enzymes.
Results of ingenol mebutate gel in actinic keratoses
Ingenol mebutate gel commonly causes skin reactions at the application site, such as:
- Pain
- Chop
- Infection
- Swelling.
Anaphylaxis and severe allergic contact dermatitis they have rarely been reported. Some patients have complained of upper respiratory symptoms and headache.
The periocular area is not suitable for ingenol mebutate gel because severe eye pain, swelling, and drooping of the eyelid may occur if the gel comes in contact with these sites. If accidental exposure occurs, flush eyes with water and seek medical attention.
Effects of ingenol mebutate gel used for facial actinic keratoses
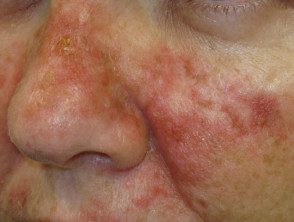
Day 3
Use of ingenol mebutate gel in pregnancy
There are no adequate and well-controlled studies of ingenol mebutate gel in pregnant women. Ingenol mebutate gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Use of ingenol mebutate gel in children
The safety and efficacy of ingenol mebutate gel in patients younger than 18 years have not been established, but actinic keratoses are generally not seen in children.
Future considerations for ingenol mebutate gel
Topical ingenol mebutate is currently in phase II clinical trials to eradicate basal cell carcinoma and scaly cell carcinoma (SCC) in the place (also called intraepidermal SCC or Bowen's disease).
Off-label use
A case report described the resolution of a keloid after treatment with 0.05% ingenol mebutate gel daily for two days.
Two out of four patients treated for actinic cheilitis had clearance.

