What is chloroquine?
Chloroquine is a synthetic antimalarial drug that was first developed in 1934. In addition to its antimalarial effects, chloroquine has been widely used in rheumatology and dermatology since the 1950s.
Despite its early successes, the use of chloroquine has been largely replaced by hydroxychloroquine, a safer hydroxylate analog term, and its availability is now limited.
Chloroquine has immunomodulator and antiinflammatory effects and can also have a sunscreen effect [1].
Who uses chloroquine?
Chloroquine and hydroxychloroquine have the same indications and use, although the dosage regimens are different. Direct comparisons suggest similar responses with equivalent doses, but a larger retina toxicity with chloroquine [2].
In dermatology, antimalarial drugs, such as chloroquine, are considered adequate treatments for:
- Lupus erythematosus
- Lattice erythematous mucinosis
- Cutaneous porphyria delays
- Chronic ulcerative stomatitis
- Sarcoidosis
-
Dermatomyositis
Antimalarial drugs are also sometimes used to treat other inflammatory skin conditions. [3].
Skin conditions that can be treated with chloroquine.
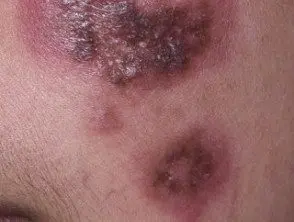
Discoid lupus erythematosus
Reticular erythematous mucinosis
Cutaneous porphyria delays
Which are the contraindications with chloroquine?
Children under 6 years of age should not receive chloroquine due to the risk of overdose and acute toxicity.
The chloroquine dose should be reduced in patients with renal disease, since up to 70% of chloroquine is excreted unchanged in the urine. Chloroquine itself can reduce kidney function in up to 10% in patients, especially in those older than 60 years. Kidney failure produces higher levels of chloroquine in the blood and therefore an increased risk of toxicity.
Chloroquine should be used with caution in patients with known delayed cutaneous porphyria. Chloroquine can be used to treat late cutaneous porphyria, but in a very low dose (125 mg twice a week) since a dose of 250 mg / day can trigger a porphyria crisis, which can be fatal.
Chloroquine is contraindicated where there is an acquaintance hypersensitivity to 4-aminoquinoline compounds.
Chloroquine crosses the placenta and is also found in low levels in breast milk, so pregnancy and lactation are often listed as contraindications to its use (see DermNet NZ pages on Safety of medications taken during pregnancy. and on Lactation and the skin). However, effects on the fetus and baby have rarely been reported, and chloroquine has been continued during pregnancy and lactation when required for malaria.
Pre-existing retinopathy (damage to the retina) and other eye problems, such as macular degeneration (blurred vision in the center of the eye) and waterfalls, make monitoring difficult, and therefore may also be relative contraindications.
Although chloroquine can occasionally trigger an outbreak of psoriasis, this is not absolute. contraindication, and the use of the drug should be decided in individual cases.
Tell me more about chloroquine
Chloroquine has similar pharmacokinetics to hydroxychloroquine. After identical doses, tissue chloroquine levels are 2.5 times higher than those observed with hydroxychloroquine. Chloroquine is rapidly and almost completely absorbed from the intestine after oral administration.
Peak plasma Chloroquine concentrations are reached in 4 to 12 hours, but plasma concentrations take 4 to 6 weeks to stabilize; therefore, it will take 2-3 months to see a therapeutic effect. Up to 70% of chloroquine is excreted unchanged through the kidneys and up to 40% is metabolized in the liver into an active metabolite.
Chloroquine binds to plasma proteins that are deposited in tissues, such as the liver, spleen, kidneys, and lungs. Chloroquine has a particularly high affinity for melanincontaining cells and therefore there are very high levels of chloroquine in the skin (mainly the epidermis) and retina. Chloroquine has a long half-life, which can range from 74 hours to 50 days, depending on cumulative dose.
The medicine can remain on the skin for 6 to 7 months after cessation of therapy
Chloroquine dosage
Chloroquine phosphate is dispensed as a 250 mg tablet, which is equivalent to 155 mg of chloroquine base.
Long-term use of chloroquine should target <2.3 mg / kg / día en función del peso corporal real del paciente. Dado el tamaño de la tableta, esto generalmente significa que debe usarse de manera intermitente; es decir, varias veces por semana en lugar de diariamente. Anteriormente, la dosis diaria máxima se calculaba sobre el peso corporal ideal del paciente y se recomendaba que la dosis acumulada fuera inferior a 460 g. Estas pautas han sido revisadas.
In delayed cutaneous porphyria, the dose should not exceed 125 mg twice a week.
Chloroquine can be used together with quinacrine, but should not be used with hydroxychloroquine.
Chloroquine is not marketed in Australia, where it is only available through the Therapeutic Goods Administration Special Access Scheme. It is no longer approved for use in New Zealand, but may be available in Section 29 (see Medsafe's information on non-approved drugs).
Dosage for hepatic disability
No dosage adjustment of chloroquine for liver failure is provided on the manufacturer's label.
Dosage for kidney failure
The following guideline is provided for the dosage of chloroquine in cases of renal failure:
- Creatinine clearance ≥10 ml / minute: no dose adjustment necessary
- Creatinine clearance <10 ml >
Which are the ocular side effects and risks of chloroquine?
The two main ocular effects of chloroquine are reversible corneal deposits and irreversible retinal toxicity.
Corneal deposits
Corneal deposits occur rapidly in the 90% of patients taking chloroquine. They are usually asymptomatic; however, patients may experience transient halos and increased sensitivity to light.
Finding corneal deposits on examination does not mean that chloroquine should be stopped.
Deposits are dose related, occur 1–1.5 months after therapy is started, and resolve after chloroquine discontinuation.
Retinal toxicity
Irreversible retinal toxicity, which causes bilateral Chloroquine ox eye retinopathy has been recognized for many years. This is a late-stage finding and should not be seen if regular monitoring is performed. The high affinity of chloroquine for melanin-containing cells, such as those found in the retina pigment epithelium, is supposed to be the cause.
Patients with antimalarial-induced retinal toxicity may have no visual symptoms initially and will only develop clinical symptoms in the later stages.
Stopping the medication when definitive signs of early retinopathy are found is important, as the chances of vision loss will generally decrease. Chloroquine should only be discontinued if there are definitive signs of retinal toxicity; Possible toxicity should be re-evaluated after several months with continued drug use. Continued use of chloroquine after a finding of definitive retinopathy results in irreversible visual damage, including effects on visual acuity, peripheral night vision and vision.
This retinopathy does not reverse, even with the cessation of chloroquine, and there is no treatment. The risk of retinal toxicity is higher with chloroquine compared to other antimalarials [4].
In 2016, with new scientific data, the American Academy of Ophthalmology revised its screening recommendations for chloroquine patients for more than 1 year. [5]. The two most important risk factors for retinopathy are the duration of treatment with chloroquine and a daily dose of chloroquine> 2.3 mg / kg of the patient's actual body weight.
Monitoring is of particular importance in patients older than 60 years and in those with kidney failure.
Detection recommendations
All patients planning to take chloroquine for at least 12 months should undergo a base eye exam within 6 months of starting chloroquine and then annually. A fundus examination alone is not enough detection; The examination should include automated visual field tests and spectral domain optical coherence tomography [6].
Other side effects
Cutaneous side effects
Chloroquine can cause:
-
Bluish gray pigmentationalthough less frequently than with quinacrine
- Urticaria
- Exfoliative dermatitis
- Psoriasiform eruption or outbreak of psoriasis
- Alopecia
- Photosensitivity
- Erythema cancel centrifugal
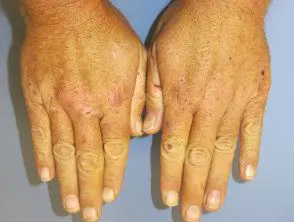
- Transverse pigmented nail bands.
Gastrointestinal side effects
Chloroquine can cause nausea, vomiting, and diarrhea; These side effects are often transient or resolve with dose reduction. Symptoms can be minimized by taking the medication with food.
Hematological effects
Hemolytic anemia and reduced blood cell count occurs very rarely at the recommended doses of chloroquine, but may occasionally occur in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Neuromuscular effects
Neuromyopathy (diseases of the nerves and muscle tissues), myalgia, and fatigue has been reported with the use of chloroquine. Therefore, care should be taken in patients with myasthenia gravis and dermatomyositis.
Pharmacological interactions with antimalarial drugs
The following medications should be reviewed when used concomitantly with chloroquine:
- Digoxin
- Methotrexate
- D-penicillamine
- Cyclosporine
- Beta blockers
- Amiodarone
- Penicillin
- Cimetidine
- Ritonavir
- Cholestyramine
- Antacids
- Aminoglycosides
- Corticosteroids
- Neostigmine
- Physostigmine.
Of smoking
Smoking is reported inhibit cytochrome P450 enzyme system, decreasing the effectiveness of chloroquine [7].
What monitoring is required with chloroquine?
- The patient's complete blood count should be checked before treatment and monthly for the first 3 months, then every 4–6 months. [8].
- The patient's kidney function should be checked at baseline, after 1 month, after 3 months, and then every 4–6 months to assess any changes in the risk of adverse eye effects (more frequent monitoring is required if laboratory values are abnormal or in high-risk patients) [8].
- A pregnancy test should be performed in women of childbearing age at the start of the study and the patient should understand the need for reliable use of contraceptives during therapy.
- Consider testing for G6PD deficiency, especially in people from Africa, Asia, Oceania, or southern Europe.
- All patients should undergo an initial eye exam within the first 6 months of starting chloroquine if treatment is anticipated to be long-term. In the absence of specific risk factors, an annual evaluation should be performed.

