What is BRCA1-associated protein 1?
BRCA1-associated protein 1 (BAP1) is a nuclear protein encoded by BAP1 tumor-suppressor gene Located in chromosome 3 (locus 3p21.1). The BAP1 protein acts as a desubiquitinant. enzyme - remove ubiquitin, a regulator of protein degradation, and is involved in many keys cellular features including:
- DNA repair
- Histone desubiquitination (proteins that 'package' DNA into chromosomes)
- Chromatin remodeling (the process that opens the coils of DNA to allow gene expression)
- Transcription (the process that copies DNA into RNA to make proteins)
- Regulation of the cell cycle [1].
For more information on genes associate with melanoma, see the DermNet pages on Genes and Melanoma, Melanoma Genetics, and Genetic melanoma tests.
Who receives the germ line BAP1 mutation?
the predominance germ line BAP1 mutations in the general population it is not known. At least 215 affected individuals from 87 families have been reported in the literature to date (as of September 2018) [2].
What Causes the Germ Line? BAP1 mutation?
Germ line BAP1 mutations are inherited in a autosomal dominant pattern, meaning that only one parent has to carry the mutated gene for their child to inherit the condition. Each somatic cell of affected offspring carries a mutant BAP1 allele and a normal wild-type allele. An additional of somatic mutation must be acquired on the wild-type allele for the cell to lose BAP1 tumor suppressor function.
The exact genotype–phenotype correlation between location and type of mutation and Cancer the risk is not known [3]. Some mechanisms of mutagenesis have been reported, most of which result in a truncated protein [4].
Not all mutations are detrimental to protein function. Significant BAP1 Mutations are those that affect the nuclear localization sequence, resulting in retention of the protein in the cytoplasm, or the catalytic domain of carboxy-terminal ubiquitin hydrolase, which affects deubiquitination activity [2,5].
What conditions are associated with the germ line? BAP1 mutation?
In 2011, a hereditary cancer predisposition syndrome was proposed in association with the germ line BAP1 mutation [6–8]. Carriers of the mutation have a high frequency of four main malignant tumors; these are:
- Evil one mesothelioma (pleural and peritoneal)
- Uveal (ocular) melanoma
- Cutaneous melanoma
- Renal cell carcinoma.
A wide range of other tumors have been reported in association with the mutation, including an increase incidence from:
- Basal cell carcinoma
- Breast cancer
- Cholangiocarcinoma
- Meningioma
- Neuroendocrine tumors
- Thyroid cancer.
Although it has a BRCA1 protein binding site, mutations in the BAP1 gene are not common in BRCA1-associated breast cancer. More studies are needed for confirmation.
The lifetime risk of developing cancer in people with germ line mutation is not known. Large population studies are required to estimate the true incidence of cancer in those with the mutation. In the 215 reported cases, 60 individuals (28%) developed uveal melanoma, 48 mesotheliomas (22%), 38 (18%) cutaneous melanoma, and 20 (9%) renal cell carcinoma. [2]. However, the germ line BAP1 mutations represent only a small proportion of these cancers overall; approximately 1.6% [8.9] for 4% [10.11] from uveal melanomas, 1–2% [12] for malignant mesothelioma, 0.01% [13] to 0.63% [6,10] for cutaneous melanoma, and 1.2% [14] for renal cell carcinomas.
Cancer in BAP1 mutation carriers are reported at a younger median age of onset and have a variation forecast significance compared to the general population (Table 1). Somatic and germ line BAP1 loss is associated with a more aggressive phenotype in uveal melanoma [9,15,16] and renal cell carcinoma [17,18], possibly shorter disease-free survival, and melanoma-specific survival in cutaneous melanoma [19], but better overall survival in mesothelioma [20–22].
| Type of cancer | Mean age of onset in BAP1 mutant germline (range) [3] | Mean age of onset (general population) [3] | Frequency (%) in a cohort of 215 patients with a positive germline BAP1 mutation [2] | Forecast germline-associated tumors BAP1 |
|---|---|---|---|---|
| Uveal melanoma | 51 (16–72) | 62 | 28 | Worst |
| Malignant mesothelioma | 53 (34–85) | 74 | 22 | Best |
| Cutaneous melanoma | 45 (21 or 25–72) | 58 | 18 years | Possibly worse |
| Renal cell carcinoma | 47 (36–72) | 64 | 9 9 | Worst |
What is a BAP1-inactivated melanocytic tumor?
the BAP1Inactivated melanocytic tumor is a rare type of melanocytic nevus and it is one of the earliest and most common clinical manifestations of the germ line BAP1 mutation. The tumor is characterized by the loss of BAP1 expression on immunoperoxidase staining; this was first described by Thomas Wiesner in 2011 [6]. BAP1Non-activated melanocytic tumors occur at approximately 67-75% of the germ line BAP1 carriers, typically after the second decade of life [2,5]. These tumors were initially histologically classified as atypical Spitz tumors due to overlapping characteristics between Spitz naevi and spitzoid melanoma, but at a molecular level, BAP1 loss and BRAF The mutation is not seen in most atypical Spitz tumors.
At the World Health Organization 2018 WHO classification of skin tumors, BAP1-nactivated (4th Ed, 2018), BAP1 inactivated melanocytic tumors are classified as BAP1-nevus inactivated and BAP1-Inactivated melanocytoma (if there are atypical features) [23].
Other names include BAP-oma, Wiesner naevus, melanocytic BAP1-atypical mutant intradermal nevoid melanocytic melanoma and tumor proliferation.
BAP1-inactivated melanocytic tumors may be sporadic in individuals without BAP1 mutation or germ line BAP1 family syndrome [6].
What are the clinical characteristics of BAP1inactivated melanocytic tumor?
BAP1Activated melanocytic tumors appear from the second to third decade of life. Are circumscribed pink, orange, tan, or skin-colored papules, with an average size of 5 mm. Other features include:
- A dome shape o pedunculated morphology
- A clinical similarity to dermal naevi
- Five to 50 injuries per individual
- A typical location on the scalp and trunk.
BAP1inactivated melanocytic tumor
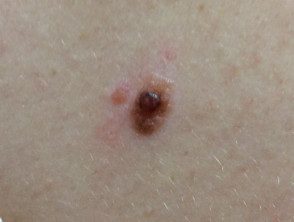
BAP-oma
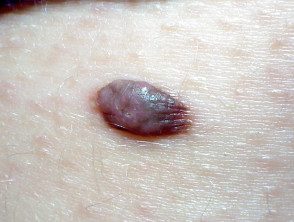
BAP-oma
What are the dermoscopic characteristics of BAP1inactivated melanocytic tumor?
Dermoscopy of BAP1inactivated melanocytic tumor is not well described (only case reports).
- There may be an unstructured pink central area, with a variety of distribution of peripheral pigmented globules
- There may be polymorphous vessels centrally [2].
- Some injuries have a central asymmetric multicomponent pattern with a peripheral lattice globular pattern, which may reflect the adjacent common component of nevi from which the tumor can evolve [23,24].
- Other lesions have an unstructured whitish central area with irregular brownish-gray dots or spots and irregular peripherals, linear vessels [24].
Dermoscopy of BAP1inactivated melanocytic tumor
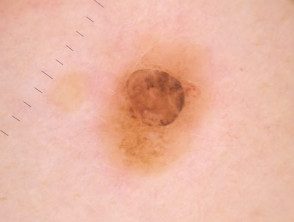
BAP-oma dermoscopy
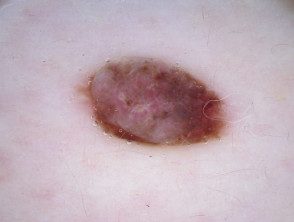
BAP-oma dermoscopy
Which are the histological characteristics of BAP1inactivated melanocytic tumor?
A BAP1Inactivated melanocytic tumor is predominantly based on the dermis (figure a), but it can be combined with a smaller adjacent attachment area that has more regular nevoid cells (representing a common nevus component).
- BAP1Non-activated melanocytic tumors are composed mainly of large epithelioid (or spitzoid) melanocytes with copious eosinophilic cytoplasm and large vesicular nuclei (figure b, which also shows surrounding smaller regular melanocytes, and figure c).
- Spitzoid features include cytologic marking atypia with nuclear pleomorphism, hyperchromatismand predominant nucleoli; however, these tumors lack the bodies of Kamino and the spindle-shaped melanocytes, which are generally seen in Spitz nevi [25].
- To reflect the spectrum of atypia demonstrated by BAP1Inactivated skin tumors, WHO terminology classifies those with benign-apparent navoid cells and minimal atypia like BAP1activated nevi, and those with highly atypical large epithelioid cells and marked pleomorphism as BAP1 melanocytomas [23].
- There is low mitotic tumor activity and infiltration lymphocytes are often present
A BAP1-inactivated melanocytic tumor is BAP1 negative (biallelic loss) and has melanocytes. Please note that there is a loss of BAP1 expression in immunohistochemistry (IHC) of larger epithelioid melanocytes, while regular melanocytes retain BAP1 expression (figure d).
Figure 1. Histopathology of BAP1inactivated melanocytic tumor
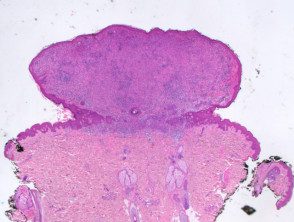
to. Low power view, hematoxylin and eosin staining
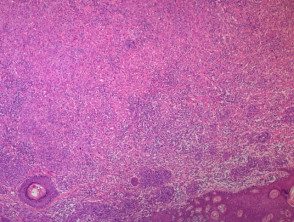
yes. x 4, hematoxylin and eosin staining
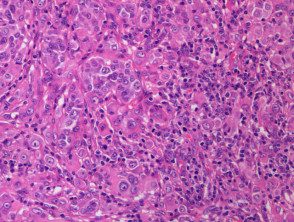
C. x 20, hemotoxilin and eosin staining
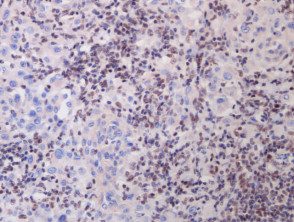
re. x 20, immunohistochemistry
BAP1-deficient injury. Man 14, back with a pedunculated lesion. Images provided by Professor Richard Scolyer.
What is the malignant potential of the BAP1-inactivated melanocytic tumor?
Very little is known about the natural history of BAP1activated melanocytic tumor phenotypic or histological characteristics of primary cutaneous melanoma in this cohort. Despite the high count of injuries in patients with BAP1Inactivated melanocytic tumors, there is a low rate of cutaneous melanoma [2]. Biallelic BAP1 loss and BRAF the mutation is not sufficient for the development of melanoma [6]. There have been some reports of the transformation of a BAP1Melanocytic tumor inactivated to melanoma [26]. Depending on the degree of atypia, the lesions can be classified as having uncertain malignant potential. They usually have a indolent course [27].
How is a BAP1-Managed inactivated melanocytic tumor?
There are no prescriptive guidelines for the management of BAP1-Inactivated melanocytic tumor. Any identified BAP1The inactivated melanocytic tumor should be followed closely with sequential digital dermoscopic images and removed if a change occurs. If excised, complete excision with histologically clear margins is mandatory. Consideration should be given to discussing lesions with uncertain malignant potential in a multidisciplinary melanoma meeting.
Who should be considered for genetic screening?
Indications for genetic testing include a strong personal or family history of all four major malignancies, along with multiple melanocytic nevi that resemble BAP1Inactivated melanocytic tumors or atypical epithelioid tumors with spitzoid characteristics in pathology [3,27].
Patients should be referred for genetic counseling prior to the decision to test for the genetic mutation. When referring you for testing, your doctor should keep in mind that there are currently no formalized cancer surveillance plans for patients diagnosed as germline positive. BAP1 mutation.
What is the germ line like? BAP1 diagnosed mutation?
The mutation detection test involves direct (Sanger) sequencing of blood or salivary DNA and direct sequencing of tumor tissue. BAP1Melanocytic tumor tissue or other inactivated tumor tissue can be examined for loss of BAP1 nuclear staining in IHC as it is highly sensitive and specific [27]. However, there is a risk of false negatives. Loss of two BAP1 alleles is required for the test to show a total lack of BAP1 expression. Melanocytic nevi in the germ line BAP1-mutants cannot be BAP1 deficient in IHC staining as they retain a wild-type allele. Cells with mono-allelic inactivation will have nuclear staining but may also show cytoplasmic staining for BAP1 if the nuclear localization sequence is affected [2]. Selecting a nevus that looks like a BAP1Internal positive or inactivated melanocytic tumor control you can reduce this risk.
Note that BAP1Deficient nevi may be present in individuals without the germline mutation (due to a somatic cause with two strokes confined to tumor tissue). Formal genetic testing is always required to diagnose the germline mutation.
What is the management of the germ line? BAP1-positive patient?
There are no formal or evidence-based cancer surveillance guidelines for patients with the germline BAP1 mutation. Currently proposed surveillance recommendations include screening for uveal melanoma, cutaneous melanoma, renal cell carcinoma, and mesothelioma. [1,3,5,20,28–30].
Uveal melanoma
An annual review with a ophthalmologist Specializing in uveal melanoma is recommended, beginning at age 16 (a younger age may be considered if a family member has been diagnosed with early uveal melanoma). Some suggest starting screening at 11 years of age, which is five years younger than the earliest reported BAP1associated uveal melanoma [3,28]. A biannual ophthalmological exam is recommended starting at the age of 30. The examination should consist of indirect ophthalmoscopy with a well dilated pupil, wide field fundus photography and eyepiece ultrasound.
Cutaneous melanoma
A comprehensive skin exam twice a year should be performed by a dermatologist specialized in melanoma, which involves a systematic review of whole body photography from 18 years of age. Sequential digital dermoscopic examination or excision of any mildly irregular or BAP1A lesion similar to a deactivated melanocytic tumor is recommended. Sun protection should also be emphasized.
Renal cell carcinoma and mesothelioma
The annual evaluation should begin between the ages of 30 and 55 with a clinical abdominal and respiratory examination looking for abdominal masses, distention, ascitesor signs of a Pleural effusion.
- The patient may have the option of investigating symptoms or asymptomatic image surveillance every two years. Imaging involves ultrasound of the chest and renal tract and consideration of magnetic resonance image (Magnetic resonance) to investigate pleural or peritoneal disease.
- After age 55, consider adding abdomen / chest computed tomography (Connecticut) with contrast every two years as an alternative to magnetic resonance imaging.
- Consider following the established protocol for von Hippel-Lindau disease, which involves annual abdominal / renal ultrasound and computed tomography or MRI every two years [3].
-
Smoking and exposure to asbestos should be avoided.
What are the future treatment options for advanced patients BAP1related to cancer?
the therapeutic benefit of drugs targeting different parts of the BAP1 path is under investigation for BAP1-Mutant cancers Currently there are Phase II and III clinical trials of the histone deacetylase inhibitor vorinostat (HDACi) and the enhancer zeste homolog 2 (EZH2) inhibitors (e.g., Tazemetosat) for patients with advanced pleural mesothelioma and metastatic uveal melanoma. waste of BAP1 leads to abnormal ubiquitination of histone H2A and overexpression of EZH2, which HDAC and EZH2 can counteract, respectively [5].
Thanks
We would like to thank clinical geneticist Dr. Annabel Goodwin, A / Prof R Max Conway, and Dr. Rony Kapoor for their discussion of cancer incidence and surveillance issues in this cohort, and co-authorship in recent BAP1 surveillance proposal [30].

