Acquired melanocytic nevus
Acquired melanoyctic nevus or mole is common benign tumor, which usually appears during childhood and adolescence. Sun exposure is a causal factor, particularly in childhood. A point mutation at BRAF gene (most often v600E) is usually the starter genetic mutation. The lesions evolve with age, the initial injury being macular with nests proliferating melanocytes confined to the dermoepidermal junction. Over time, the nests spread towards the dermis and injuries increase. With further maturation of the junctional activity, it ceases and the nevus becomes intradermal.
Many melanocytic naevi eventually returns and the number of nevi decreases with the age of 50 years.
The classification of common acquired nevi is based primarily on the location of the nests (e.g., union, compound, dermal or combined nevi) leading to features that are often clinically recognizable. Dermatoscopy is also useful in classifying nevi, especially when there are atypical clinical features that raise suspicion of melanoma.
Union nevi
Junctional nevi are generally pigmented macules in clinical examination. Histologically, proliferating melanocytes are found at the dermoepidermal junction. The cells within the nests are oval or cuboidal in shape, with a clear cytoplasm and variable pigmentation (Figure 1).
Junctional melanocytic nevus pathology
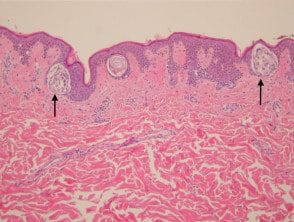
Figure 1
Compound nevus
Compound melanocytic nevi have a raised central area with surrounding flat pigmentation. the epidermis may be normal in appearance, acanthotic or visualization seborrheic keratosis-like changes. Melanocyte nests are found at the dermoepidermal junction and within the dermis. Melanocytes exhibit maturation as they become deeper and tend to be smaller with less pigmentation. (Figures 2, 3).
Melanocytic pathology composed of nevus
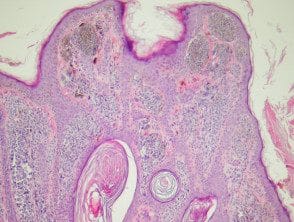
Figure 2
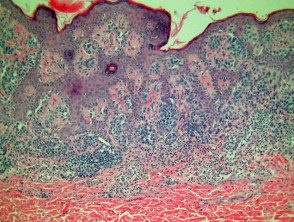
figure 3
Intradermal nevi
Intradermal nevi are dome-shaped, nodular or polypoid lesions that may become unpigmented, particularly on the face. Melanocyte nests are confined to the dermis. Melanocytes can show 'pseudo-inclusions', which are invaginations of cytoplasm in the core giving the appearance of nuclear inclusions or cells with multiple nuclei. Deeper, the cells of the nevus may become spindle-shaped or “neurotized.” Fatty differentiation It is not strange. Bone formation is a very rare finding in common nevi (Nanta nevi) (figure 4).
Pathology of intradermal nevus
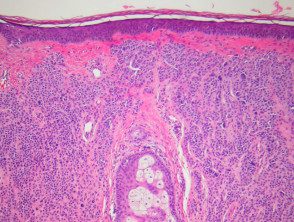
Figure 4
Meyerson naevus
Meyerson's neevus has a eczematous halo surrounding a junctional, compound, or intradermal nevus. There's a concurrent subacute finding spongy dermatitis with a melanocytic nevus (figure 5).
Meyerson naevus pathology
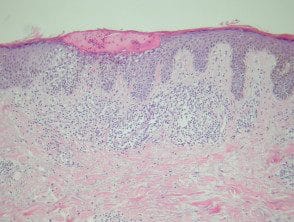
Figure 5
Balloon cell nevus
Balloon cells are large nevus cells with clear cytoplasm. A balloon cell nevus is usually diagnosed when more than half of all nevus cells within a lesion are balloon cells. Clinically these lesions are not distinctive (figure 6).
Nevus balloon cell pathology
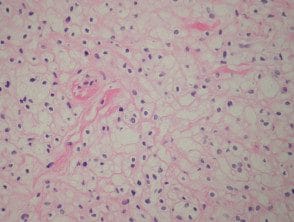
Figure 6
Returning naevi
Melanocytic nevi may eventually return; nevus cells being replaced by collagen, fat, elastin and soil substance (figure 7, nevus with fat and neural metaplasia)
Regression can be announced by lymphocytic Destruction of nevus cells giving rise to a nevus halo. In these lesions, the dermis shows dense lymphocytes. infiltrators surrounding nests of melanocytes (figures 8, 9). Due to the infiltrateObfuscation of the melanocytic lesion, evaluation of the cytology Sometimes it can be a challenge. Clinically, there is a halo of depigmentation surrounding one or more nevi. Halo naevi may be associated with vitiligo.
Regression of melanocytic pathology of nevi
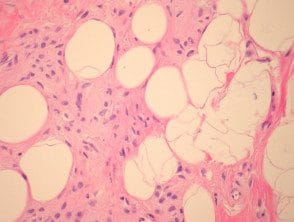
Figure 7
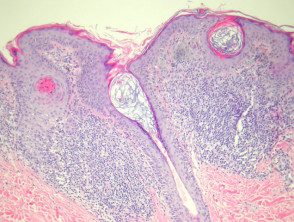
Figure 8
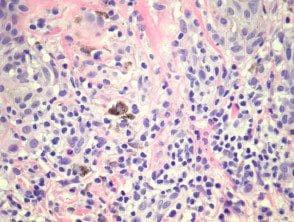
Figure 9
Site-specific characteristics of melanocytic nevi
Benign melanocytic naevi at various sites may show unusual histopathological characteristics that can mimic melanoma.
Nevi of the auricular region, breast, conjunctiva and the ankle sometimes have an atypical appearance proliferation of melanocytes in the epidermis, often with pagetosis and cytology atypia. Some features seen in dysplastic nevus as lamellar fibroplasia of the superficial dermis can be noticed. Clues to a benign diagnosis include clear circumscription and maturation with descent into the dermis. It is important that these lesions do not undergo a partial biopsy, as interpretation can be difficult. Partial biopsies may fail to demonstrate reassuring characteristics (particularly discreet side district).
Genital nevi
Atypical genital naevi are probably the special sites most frequently diagnosed as melanoma (Figure 10). Obviously, this can lead to important morbidity if mutilation surgery is performed to achieve wide margins. Lichen sclerosus superimposed on a genital nevus is a classic simulator of evil one melanoma. Clinical correlation can be very useful in these cases.
Genital pathology of melanocytic nevus
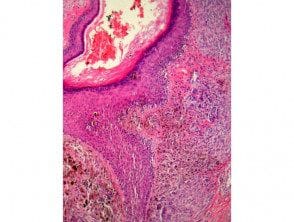
Figure 10
Acral naevi
Melanocytic acral nevi often show quite marked Pagetosis, sometimes at the upper levels of the epidermis, which may mimic melanoma. in the place. The acronym MANIAC (Melanocytic Acral Naevi with Intraepidermal Ascent of Cells) has been applied to describe these benign nevi. A key useful feature is the primarily nested nature of the lesion with the pagetoid spotlights that arise over these nests instead of a diffuse lentiginous proliferation usually seen in acral melanoma. Discrete areas of melanin You can see “chimneys” in the stratum corneum on these nests, as opposed to the diffuse corneal melanin statement seen in melanoma. Additionally, acral nevi should show some dermal maturation if there is a dermal component (Figures 11, 12).
Pathology of acral melanocytic nevi
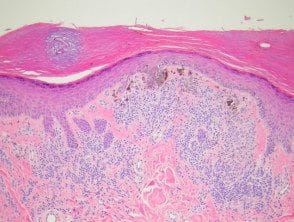
Figure 11
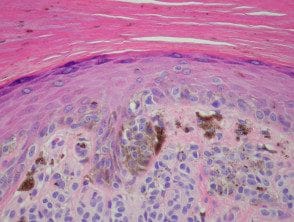
Figure 12
Dysplastic nevi
Dysplastic nevus is also called Clark nevus. It is a rather controversial entity: some authorities demand that the term be eliminated from the dermatopathological lexicon. Dysplastic or Clark's nevi are benign, often large moles with unusual clinical features and characteristics. histological characteristics. Although it is generally sporadic, there are family cases (dysplastic nevi syndrome)
Histologically, dysplastic nevi have the following characteristic features (figure 13):
- Intraepidermal lentigo hyperplasia of melanocytes with proliferation of individual melanocytes and basal nests
- melanocyte cytological atypia with enlargement hyperchromatic prominent nuclei and nucleoli. Some pathologists rate the degree of atypia as mild, moderate, or severe (although there are no diagnostic guidelines for this).
- Stromal response with fibroplasia (scarring) of the papillary dermis and dermal proliferation of dendrocytes. There may also be fibrosis from the top lattice dermis indicating regression.
- Architectural atypia including the “shoulder phenomenon,” where the nesting of the junction extends more widely than the dermal component.
Dysplastic pathology of melanocytic nevus
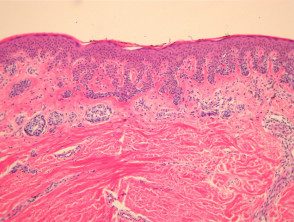
Figure 13
Superficial atypical melanocytic proliferations of unknown meaning
Some pathologists use the term Superficial Atypical Melanocytic Proliferation of Unknown Significance or SAMPUS to describe dysplastic melanocytic lesions in which it is difficult to exclude melanoma in situ or the radial growth phase. invader melanoma. SAMPUS is often applied when a conclusive diagnosis cannot be reached. The term should ideally be avoided.
However, the histological changes of SAMPUS overlap with dysplastic nevi (lentiginous proliferation of melanocytes with cytological atypia). focal pagetoid spread of single or nested melanocytes (figures 14, 15).
Superficial atypical melanocytic proliferations of pathology of unknown significance (SAMPUS)
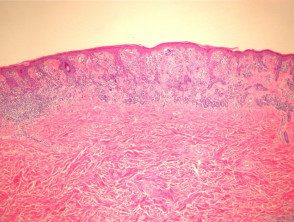
Figure 14
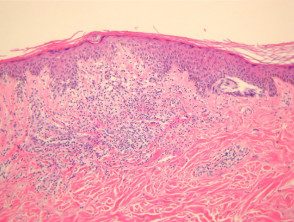
Figure 15
Differential diagnosis
The most important differential is melanoma, but other forms of melanocytic nevi such as congenital neevi, Spitz naevi or blue neevi may need to be considered.

